Abstract
Objectives
Diffuse large B-cell lymphoma (DLBCL) is a disease with highly heterogeneous molecular characteristics and clinical course. Refractory/relapsed DLBCL shows poor prognosis, and new therapeutic approaches are urgently needed to improve its clinical outcomes. It is important to investigate the mechanism of development of DLBCL, in order to discover new molecular therapeutic targets. In this study, Cytometry by Time-Of-Flight mass spectrometry (CyTOF) was used to profile the immune cells in peripheral blood of DLBCL patients, to investigate the changes of immune cell subpopulation, and to find possible new targets for immune therapy of DLBCL.
Methods
Two CyTOF antibody panels targeting innate and adaptive immune cells, respectively, were designed to detect the characteristics of immune cell subpopulation. The antibodies include cell surface, intracellular and nuclear markers, targeting immune-related functions such as cell phenotyping, activation, proliferation, immune checkpoints, and chemokine receptors. Peripheral blood was collected from 50 DLBCL patients and 14 healthy controls. After staining and data collection, dimensional reduction such as viSNE and clustering such as SPADE and PARC were performed in Cytobank, OMIQ and R to comprehensively compare the differences in cell subset composition and function between patients and healthy controls.
Results
CD8+ T cells were more differentiated into effector phenotype with senescence and exhausted characteristics in DLBCL patients
CD8+ T cells decreased significantly in DLBCL patients. Percentages of effector memory (EM) and CD45RA+ effector memory cells (EMRA) in CD8+ T cells were elevated in DLBCL patients. The higher expression intensities of CD45RO, KLRG1, PD-1, CTLA-4, and TIGIT in CD8+T cells imply the senescence and exhausted characteristics of CD8+ T cells in patients.
After clustering analysis by SPADE, CD8+ T cells could be divided into 4 clusters, among which the proportion of CD45RO+KLRG1+ cluster was significantly higher in patients. This subset highly expressed PD-1, CTLA-4, TIGIT, GITR, CD25, and FoxP3, suggesting that they are exhausted effector CD8+ T cells with immunosuppressive phenotype. Proportions of the other 3 clusters, which were CD27+CCR7+ naïve CD8+ T cells, Granzyme B+HLA-DR+Ki-67+ central memory CD8+ T cells, and Granzyme B+HLA-DR+ central memory CD8+ T cells respectively, did not change significantly.
CD56brightNK cell subset decreased significantly in DLBCL patients
NK cells decreased significantly in DLBCL patients. By PARC clustering analysis, NK cells were divided into 3 clusters. The patient had a significantly reduced CD56bright subset. While, CD56brightKi-67+ cluster as well as CD56dimCD16+ cluster did not significantly change.
Two subsets of monocytes with immunosuppressive phenotype emerged in DLBCL patients
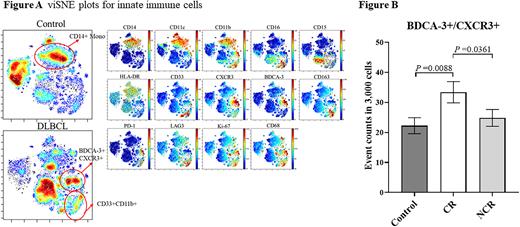
Compared with healthy controls, monocytes showed a significant elevation in DLBCL patients. However, the proportion of CD14+HLA-DR+ monocytes decreased in patients. 2 subsets of CD33+CD11b+ and BDCA3+CXCR3+ monocytes appeared, which showed higher expression of immunosuppression-related markers CD163, PD-1, and LAG3, and relatively lower expression of HLA-DR. (Figure 1) CD33+CD11b+ cells were consistent with the MDSC phenotype; whereas the proportion of BDCA3+CXCR3+ subset in total leukocytes correlated with patients' treatment response for chemotherapy. The level of BDCA3+CXCR3+ monocytes was significantly higher in patients who obtained CR after 6 cycles of chemotherapy than in patients who did not. (Figure 2)
Conclusions
By a multidimensional CyTOF staining approach, we depicted the immune cell profile of peripheral blood in DLBCL patients, analyzed the changes of the proportion of immune cell subset, and explored the changes in differentiation and function of different immune cell subsets in depth. For the first time, we found an increase in BDCA3+CXCR3+ monocyte subpopulation in DLBCL patients, and its proportion may be closely related to the prognosis of DLBCL patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal